Heat of fusion and heat of vaporization worksheet – Embarking on a journey to understand the concepts of heat of fusion and heat of vaporization, this worksheet provides a comprehensive exploration of these phenomena, their applications, and the differences that set them apart.
Delving into the intricacies of heat transfer, we will uncover the processes involved in changing a substance’s state from solid to liquid and liquid to gas, exploring real-world applications that demonstrate the significance of these concepts in our daily lives.
Heat of Fusion and Heat of Vaporization
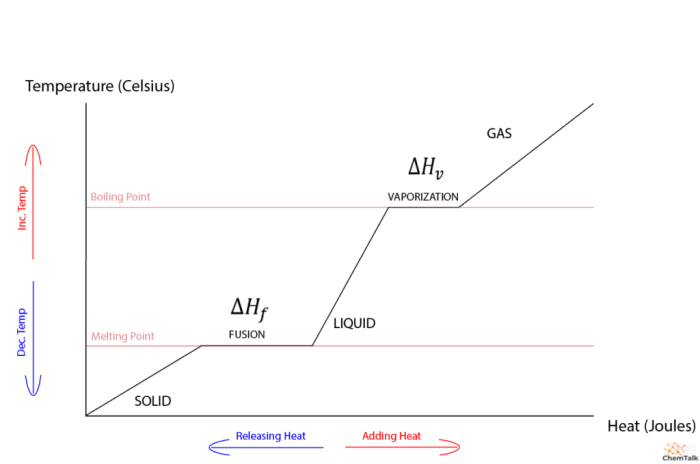
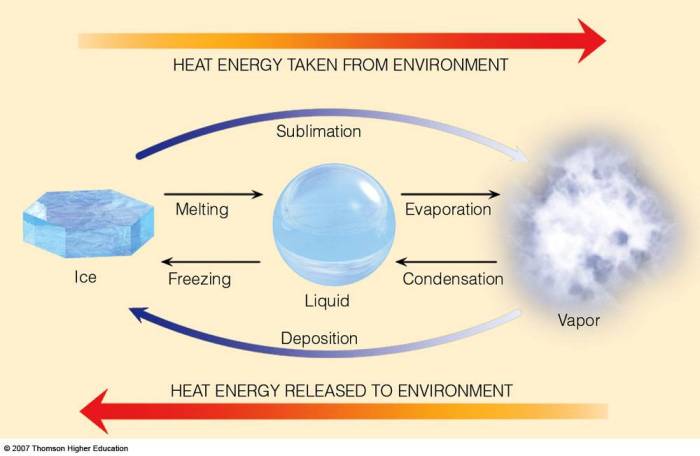
Heat of fusion and heat of vaporization are two important concepts in thermodynamics that describe the energy changes associated with phase transitions. Heat of fusion refers to the energy required to change a substance from a solid to a liquid, while heat of vaporization refers to the energy required to change a substance from a liquid to a gas.
Heat of Fusion
Heat of fusion is the amount of energy required to change one mole of a substance from a solid to a liquid at its melting point. It is a measure of the strength of the intermolecular forces in the solid phase.
Substances with strong intermolecular forces, such as metals and ionic compounds, have high heats of fusion, while substances with weak intermolecular forces, such as molecular compounds and organic compounds, have low heats of fusion.
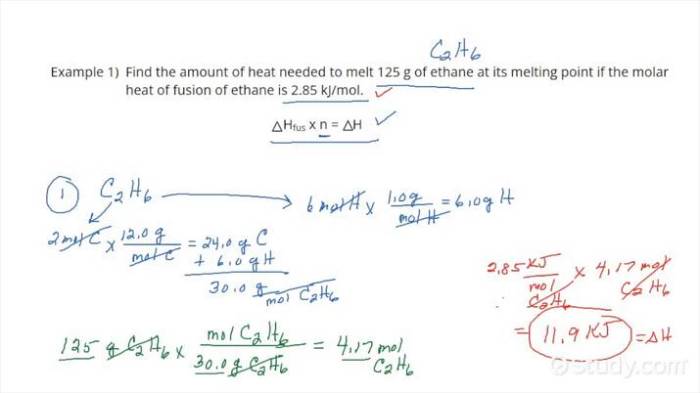
The heat of fusion can be calculated using the following equation:
$$\Delta H_fus = \fracQn$$where:
- $\Delta H_fus$ is the heat of fusion in kJ/mol
- $Q$ is the heat absorbed in kJ
- $n$ is the number of moles of the substance
The following table lists the heats of fusion for some common substances:
| Substance | Melting point (°C) | Heat of fusion (kJ/mol) |
|---|---|---|
| Water | ||
| Sodium chloride | ||
| Iron | ||
| Gold |
Heat of Vaporization
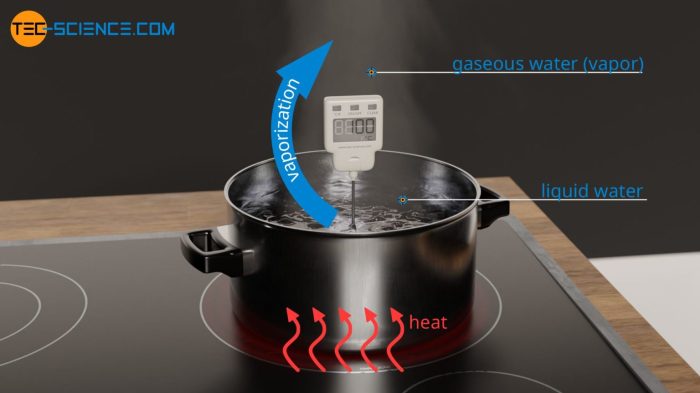
Heat of vaporization is the amount of energy required to change one mole of a substance from a liquid to a gas at its boiling point. It is a measure of the strength of the intermolecular forces in the liquid phase.
Substances with strong intermolecular forces, such as water and alcohols, have high heats of vaporization, while substances with weak intermolecular forces, such as nonpolar organic compounds, have low heats of vaporization.
The heat of vaporization can be calculated using the following equation:
$$\Delta H_vap = \fracQn$$where:
- $\Delta H_vap$ is the heat of vaporization in kJ/mol
- $Q$ is the heat absorbed in kJ
- $n$ is the number of moles of the substance
The following table lists the heats of vaporization for some common substances:
| Substance | Boiling point (°C) | Heat of vaporization (kJ/mol) |
|---|---|---|
| Water | ||
| Ethanol | ||
| Hexane | ||
| Benzene |
Comparing Heat of Fusion and Heat of Vaporization, Heat of fusion and heat of vaporization worksheet
Heat of fusion and heat of vaporization are both measures of the energy required to change the phase of a substance. However, there are some key differences between the two.
- Magnitude:Heat of vaporization is typically much larger than heat of fusion. This is because it takes more energy to break the intermolecular forces in a liquid than it does to break the intermolecular forces in a solid.
- Temperature dependence:Heat of fusion is constant at the melting point, while heat of vaporization increases with temperature. This is because the intermolecular forces in a liquid become weaker as the temperature increases.
- Pressure dependence:Heat of fusion is independent of pressure, while heat of vaporization decreases with increasing pressure. This is because the volume of a gas increases with decreasing pressure, and it takes less energy to break the intermolecular forces in a gas when the volume is larger.
The following table summarizes the key differences between heat of fusion and heat of vaporization:
| Property | Heat of fusion | Heat of vaporization |
|---|---|---|
| Magnitude | Smaller | Larger |
| Temperature dependence | Constant at melting point | Increases with temperature |
| Pressure dependence | Independent of pressure | Decreases with increasing pressure |
Applications of Heat of Fusion and Heat of Vaporization
Heat of fusion and heat of vaporization have a wide range of applications in everyday life.
Applications of heat of fusion
- Melting ice:Heat of fusion is used to melt ice and snow. This is important for transportation, safety, and comfort.
- Refrigeration:Heat of fusion is used in refrigerators and freezers to keep food cold. The refrigerant absorbs heat from the food, causing it to evaporate. The refrigerant then condenses, releasing heat to the outside environment.
- Thermal energy storage:Heat of fusion can be used to store thermal energy. This is done by melting a solid material, such as ice or salt, and then storing the liquid in an insulated container. When heat is needed, the liquid is solidified, releasing heat.
Applications of heat of vaporization
- Evaporation:Heat of vaporization is used to evaporate liquids. This is important for processes such as drying clothes, cooking food, and cooling the body.
- Condensation:Heat of vaporization is used to condense gases. This is important for processes such as air conditioning, refrigeration, and steam power plants.
- Distillation:Heat of vaporization is used to separate liquids based on their boiling points. This is important for processes such as producing alcohol, gasoline, and pharmaceuticals.
Detailed FAQs: Heat Of Fusion And Heat Of Vaporization Worksheet
What is the difference between heat of fusion and heat of vaporization?
Heat of fusion is the amount of energy required to change a substance from a solid to a liquid at its melting point, while heat of vaporization is the amount of energy required to change a substance from a liquid to a gas at its boiling point.
What are some examples of substances that undergo heat of fusion?
Examples of substances that undergo heat of fusion include ice melting into water, butter melting into a liquid, and wax melting into a liquid.
What are some applications of heat of fusion and heat of vaporization?
Applications of heat of fusion include storing thermal energy in thermal energy storage systems and using it in cooking and refrigeration. Applications of heat of vaporization include cooling systems, such as air conditioners and refrigerators, and power plants.