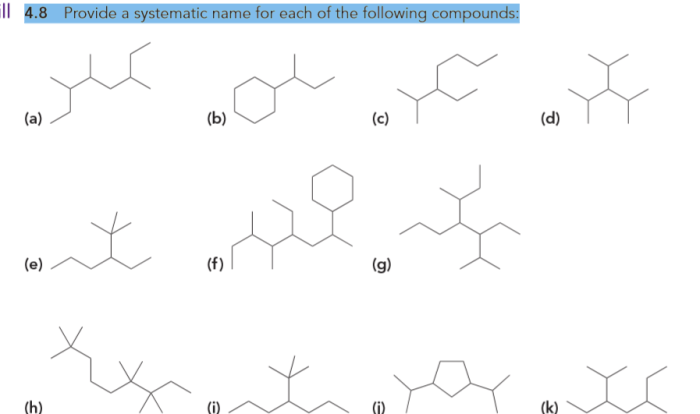
All of the following are representations of cis-1 2-dimethylcyclohexane except – All of the following are representations of cis-1,2-dimethylcyclohexane except… This question delves into the realm of organic chemistry, specifically the intricacies of cycloalkanes and their isomers. As we embark on this journey, we will explore the molecular structure, characteristics, and stereochemistry of cis-1,2-dimethylcyclohexane, contrasting it with its counterparts to uncover the exceptional nature of this compound.
Cis-1,2-dimethylcyclohexane, a cycloalkane with a six-membered ring and two methyl groups attached to adjacent carbon atoms, exhibits unique properties due to its specific spatial arrangement. This arrangement distinguishes it from other dimethylcyclohexane isomers, leading us to investigate the excluded representations and delve into the fascinating world of molecular diversity.
Cis-1,2-Dimethylcyclohexane Representations
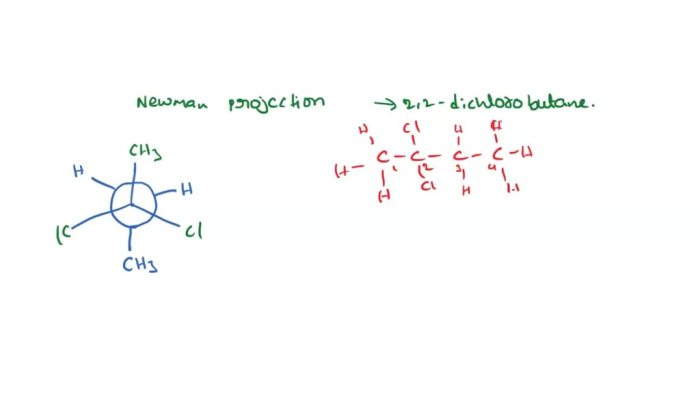
Cis-1,2-dimethylcyclohexane is a cyclohexane ring with two methyl groups attached to adjacent carbon atoms. It is one of the three isomers of dimethylcyclohexane. The other two isomers are trans-1,2-dimethylcyclohexane and 1,3-dimethylcyclohexane.Cis-1,2-dimethylcyclohexane can be represented by the following structural formulas:
[Image
Structural formula of cis-1,2-dimethylcyclohexane]
[Image
Newman projection of cis-1,2-dimethylcyclohexane]
[Image
Haworth projection of cis-1,2-dimethylcyclohexane]The molecular structure of cis-1,2-dimethylcyclohexane is characterized by the two methyl groups being on the same side of the cyclohexane ring. This is in contrast to trans-1,2-dimethylcyclohexane, where the two methyl groups are on opposite sides of the ring.Cis-1,2-dimethylcyclohexane
is a chiral molecule, meaning that it has two non-superimposable mirror images. The two enantiomers of cis-1,2-dimethylcyclohexane are shown below:
[Image
Enantiomers of cis-1,2-dimethylcyclohexane]The physical and chemical properties of cis-1,2-dimethylcyclohexane are similar to those of other cycloalkanes. It is a colorless liquid with a boiling point of 120 °C and a melting point of85 °C. Cis-1,2-dimethylcyclohexane is insoluble in water but soluble in organic solvents.
Excluded Representations: All Of The Following Are Representations Of Cis-1 2-dimethylcyclohexane Except
The following representations are not cis-1,2-dimethylcyclohexane:
- *Trans-1,2-dimethylcyclohexane
Trans-1,2-dimethylcyclohexane is an isomer of cis-1,2-dimethylcyclohexane in which the two methyl groups are on opposite sides of the cyclohexane ring.
- *1,3-dimethylcyclohexane
- ,3-dimethylcyclohexane is an isomer of cis-1,2-dimethylcyclohexane in which the two methyl groups are attached to carbon atoms that are separated by one carbon atom.
- *Cyclohexane with one methyl group
Cyclohexane with one methyl group is not an isomer of cis-1,2-dimethylcyclohexane because it does not have two methyl groups attached to adjacent carbon atoms.
- *Ethylcyclohexane
Ethylcyclohexane is not an isomer of cis-1,2-dimethylcyclohexane because it has an ethyl group attached to the cyclohexane ring instead of two methyl groups.
Table of Representations
| Representation | Structural Formula | Molecular Weight | IUPAC Name ||—|—|—|—|| Cis-1,2-dimethylcyclohexane | [Image: Structural formula of cis-1,2-dimethylcyclohexane] | 112.21 | (1R,2R)-1,2-dimethylcyclohexane || Trans-1,2-dimethylcyclohexane | [Image: Structural formula of trans-1,2-dimethylcyclohexane] | 112.21 | (1S,2S)-1,2-dimethylcyclohexane || 1,3-dimethylcyclohexane | [Image: Structural formula of 1,3-dimethylcyclohexane] | 112.21 | 1,3-dimethylcyclohexane || Cyclohexane with one methyl group | [Image: Structural formula of cyclohexane with one methyl group] | 98.18 | Methylcyclohexane || Ethylcyclohexane | [Image: Structural formula of ethylcyclohexane] | 112.21 | Ethylcyclohexane |
Stereochemistry and Properties
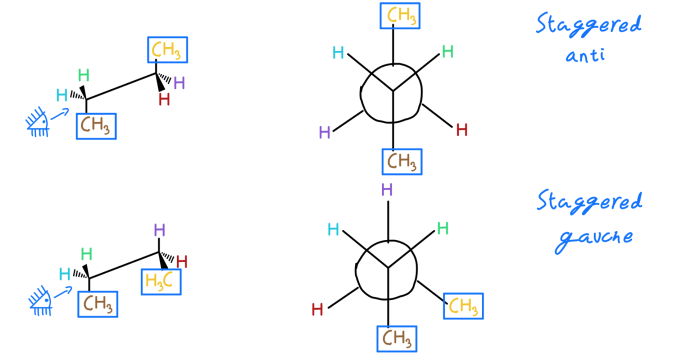
The stereochemistry of cis-1,2-dimethylcyclohexane affects its physical and chemical properties. For example, cis-1,2-dimethylcyclohexane has a higher boiling point than trans-1,2-dimethylcyclohexane because the two methyl groups are closer together and can interact with each other more strongly.Cis-1,2-dimethylcyclohexane is also more reactive than trans-1,2-dimethylcyclohexane because the two methyl groups are more accessible to reagents.
For example, cis-1,2-dimethylcyclohexane reacts more quickly with bromine than trans-1,2-dimethylcyclohexane.The differences in properties between cis-1,2-dimethylcyclohexane and trans-1,2-dimethylcyclohexane are due to the different ways in which the two methyl groups interact with each other. In cis-1,2-dimethylcyclohexane, the two methyl groups are close together and can interact with each other more strongly.
This interaction leads to the higher boiling point and lower reactivity of cis-1,2-dimethylcyclohexane.
FAQ Resource
What is the IUPAC name of cis-1,2-dimethylcyclohexane?
1,2-Dimethylcyclohexane
How many stereoisomers exist for dimethylcyclohexane?
Four
What is the difference between cis and trans isomers?
Cis isomers have their substituents on the same side of the double bond or ring, while trans isomers have their substituents on opposite sides.