Isotopes and average atomic masses worksheet – Embark on a journey into the fascinating world of isotopes and average atomic masses with this comprehensive worksheet. Designed to ignite your curiosity and deepen your understanding, this resource delves into the intricacies of isotopes, their significance, and the concept of average atomic mass.
Delve into the captivating realm of isotopes, unraveling their distinct characteristics and diverse applications. Discover the intricacies of average atomic mass, exploring its calculation and multifaceted uses. Prepare to be captivated as you navigate through exercises and engage in thought-provoking discussions, gaining invaluable insights into these fundamental chemical concepts.
Isotopes: Isotopes And Average Atomic Masses Worksheet
Isotopes are variants of an element that have the same atomic number but different numbers of neutrons. They differ in mass due to the varying neutron count, which affects the atomic mass of the element. Isotopes can be stable or radioactive, and they play a crucial role in various scientific fields, including chemistry, physics, and geology.
Significance of Isotopes
- Identify and characterize elements based on their isotopic composition
- Study nuclear reactions and radioactive decay processes
- Determine the age of geological formations using radioactive isotopes (radiometric dating)
- Trace the movement of substances in environmental and biological systems using isotopic tracers
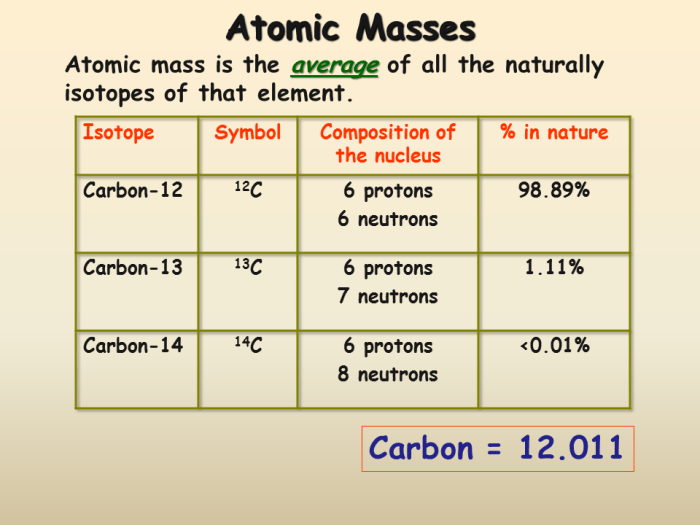
Average Atomic Mass, Isotopes and average atomic masses worksheet
Average atomic mass is the weighted average of the masses of all isotopes of an element, taking into account their relative abundances. It is expressed in atomic mass units (amu) and is used to represent the typical mass of an atom of that element.
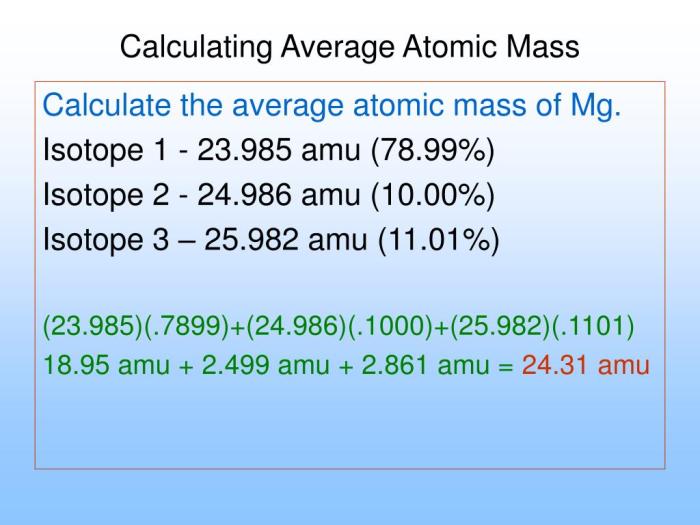
Calculation of Average Atomic Mass
The average atomic mass is calculated using the following formula:
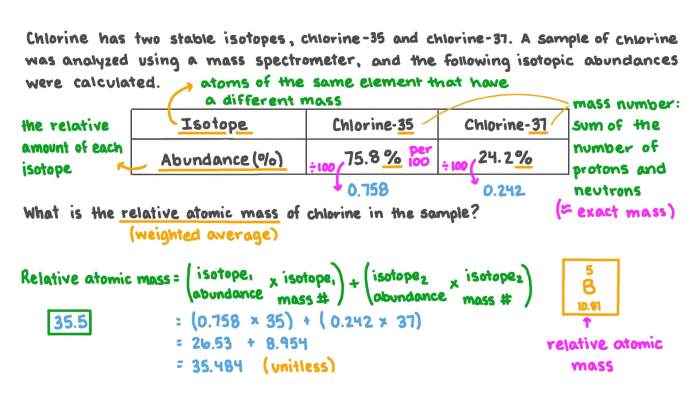
Average Atomic Mass = (Mass of Isotope 1 × Abundance of Isotope 1) + (Mass of Isotope 2 × Abundance of Isotope 2) + …
Where:
- Mass of Isotope: Mass of each isotope in atomic mass units (amu)
- Abundance of Isotope: Fractional abundance of each isotope, expressed as a percentage or decimal
Worksheet
Exercises on Isotopes and Average Atomic Mass
- Define isotopes and explain their significance.
- Describe how isotopes differ from each other and provide examples.
- Calculate the average atomic mass of an element given the masses and abundances of its isotopes.
- Explain how isotopes are used in radiometric dating and environmental tracing.
Data Table
| Isotope | Mass (amu) | Abundance (%) |
|---|---|---|
| 12C | 12.0000 | 98.93 |
| 13C | 13.0034 | 1.07 |
Discussion Questions
Importance of Isotopes in Various Fields
- Discuss the role of isotopes in understanding the structure and evolution of the universe.
- Explain how isotopes are used in medical applications, such as diagnosis and treatment.
- Explore the potential of isotopes in future technologies, such as nuclear energy and space exploration.
Challenges in Measuring Average Atomic Mass
- Identify the factors that can affect the accuracy of average atomic mass measurements.
- Discuss the limitations of using average atomic mass to represent the mass of an individual atom.
- Explore alternative methods for determining the mass of individual atoms.
Questions and Answers
What are isotopes?
Isotopes are atoms of the same element that possess an identical number of protons but differ in the number of neutrons, resulting in variations in their atomic masses.
How is average atomic mass calculated?
Average atomic mass is calculated by taking the weighted average of the atomic masses of all naturally occurring isotopes of an element, considering their relative abundances.
What are the applications of isotopes?
Isotopes find applications in diverse fields such as medicine (radioactive isotopes for diagnosis and treatment), archaeology (carbon dating), and industry (stable isotopes as tracers).